Sodium Citrate Dihydrate/ Anhydrous
Product Usage
- Mainly used as food additive and preservative
- The anticoagulant in blood transfusions
- Used to relieve discomfort in urinary tract infections
- It also works as buffering agent in food and acidity regulator as antacid
- As a sequestrant – to improve the quality and stability of the food products
- As a emulsifier – to stabilize processed foods like cheese
Sodium citrate material is derived from the citric acid (sodium salts). This material is available in the colorless granular form or powdery form. This is fragrance free material and generously mixed with water, but not in the alcohol. It is not contain any food allergens and it is suitable for consumption by vegans and vegetarians. It adds enjoyable flavor in food items. It is widely used as dehydrate salt, but it provides remarkable gain in dry products where long shelf life is needed to store it.
Citrate Derivatives Exporters
We are exemplary exporters of consistent and high quality citrate derivatives and acidulants to customers who are in business of health, beverages, pharmaceutical industries and food. Our products are qualified in domestic as well as international markets. We offer various grades products and DMF facility as per demand of clients. To know the more details or bulk order inquiry, feel free to contact our export department.
|
Technical Details |
|
|
CAS No |
6132-04-3 |
|
Mol. Formula |
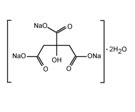
C6H5Na3O7•2H2O |
|
Mol. Weigh |
294.1 |
|
Category |
Anticoagulant |
|
Specification |
|
|
Description |
White or almost white, crystalline powder or granular crystals |
|
Solubility |
Freely soluble in water and very soluble in boiling water; insoluble in alcohol and in ether |
|
Appearance of solution |
10 % aqueous solution is clear and colourless |
|
Acidity or Alkalinity |
Passes as per the Pharmacopoeia |
|
Chlorides |
Passes as per Pharmacopoeia |
|
Sulphates |
Passes as per Pharmacopoeia |
|
Heavy metals |
Passes as per Pharmacopoeia |
|
Arsenic |
Passes as per Pharmacopoeia |
|
Oxalates |
Passes as per Pharmacopoeia |
|
Readily carbonisable substances |
Passes as per Pharmacopoeia |
|
Tartrate |
Passes as per Pharmacopoeia |
|
Pyrogens |
Passes as per Pharmacopoeia |
|
Water |
Between 11.0 and 13.0 % w/w |
|
Assay |
Between 99.0 and 100.5 % w/w |
Standard Packing: 25 KG Woven Bag / HDPE Drum Packing with double inner polyethylene bag.

